X Chromosome Inactivation is a fascinating biological process crucial to understanding genetic disorders, particularly those linked to the X chromosome. In females, the presence of two X chromosomes necessitates a complex mechanism of chromosomal silencing to ensure that gene dosage is balanced with males, who have just one X chromosome. This inactivation is not just a trivial detail; it plays a significant role in conditions such as Fragile X Syndrome and Rett Syndrome, both of which are rooted in mutations on this chromosome. Recent breakthroughs in cell biology reveal how a gelatinous, Jell-O-like substance surrounding chromosomes aids in this silencing process, supporting ongoing research into potential therapies for these challenging genetic disorders. Unlocking the secrets of X Chromosome Inactivation could ultimately pave the way for innovative treatments that resolve the underlying issues caused by X-linked mutations.
The process of X Chromosome Inactivation, often referred to as the silencing of one of the two X chromosomes in female cells, is fundamental to maintaining genetic equilibrium between sexes. This cellular phenomenon is crucial for understanding various chromosomal disorders, particularly those associated with abnormal gene expressions, such as Fragile X and Rett Syndromes. Recent studies unveil how a viscous, gelatinous material plays a pivotal role in regulating gene activity by enveloping chromosomes, thereby influencing their functional state. By delving into the intricacies of this chromosomal silencing mechanism, researchers are inching closer to potential solutions for mitigating the effects of genetic disorders. As advancements continue, the implications of these findings could herald a new era of targeted therapies aimed at correcting genetic anomalies linked to X chromosome mutations.
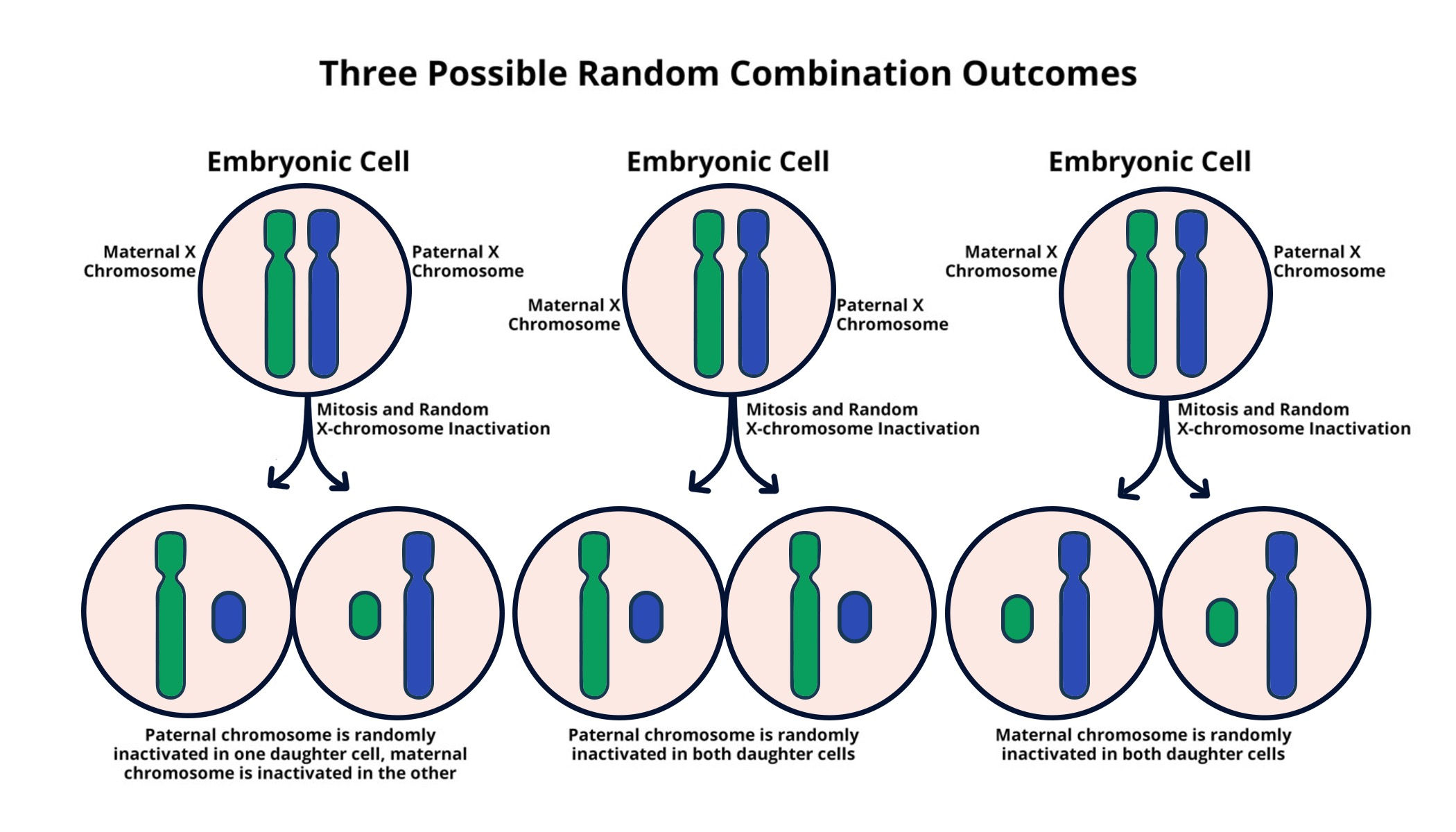
Understanding X Chromosome Inactivation
X chromosome inactivation (XCI) is a vital biological process unique to female mammals, where one of the two X chromosomes is randomly silenced. This mechanism ensures dosage compensation, balancing the expression of X-linked genes between males and females who possess differing numbers of X chromosomes. The process of XCI is essential to prevent females from having double the dosage of X-linked gene products, which could lead to detrimental effects in cellular function. Scientists, particularly in the lab of Jeannie T. Lee, have uncovered key elements governing this complex mechanism, including the role of specific RNA molecules like Xist that facilitate chromosomal silencing by modifying the surrounding chromatin structure.
The understanding of X chromosome inactivation is crucial in the context of genetic disorders that arise from mutations on the X chromosome, such as Fragile X Syndrome and Rett Syndrome. In Fragile X Syndrome, for instance, a mutation in the FMR1 gene located on the X chromosome leads to intellectual disabilities, primarily affecting males due to their single X chromosome. The research supported by the National Institutes of Health highlights the importance of cell mechanisms that not only silence genes but also potentially unsilence them, paving the way for therapeutic advancements targeting these genetic disorders.
The Role of the Gelatinous Substance in Chromosomal Silencing
Recent findings by Jeannie T. Lee’s lab have revealed that a Jell-O-like substance plays a critical role in the process of chromosomal silencing, particularly during X chromosome inactivation. This gelatinous material surrounds chromosomes and acts as a physical separator, preventing tangling and ensuring that the complex folding of DNA is maintained. As cells initiate the inactivation process, this Jell-O-like substance becomes subject to transformation by the interaction with Xist RNA, which alters its biophysical properties — making it more malleable and allowing other regulatory molecules to infiltrate it. The discovery of this process has opened new avenues of research to further investigate how chromosomal architecture affects gene expression.
Understanding the role of this gelatinous substance is also significant in the context of treating genetic disorders. For diseases like Fragile X Syndrome and Rett Syndrome, the ability to manipulate the inactivation process could mean the difference between continued silence of beneficial genes and their expression. Current research aims to decipher how releasing the silencing mechanism can restore function to mutated genes, thereby enhancing the therapeutic potential of treatments aimed at these conditions. The Jell-O-like substance thus serves not only as a structural component of chromosomes but also as a key player in the regulation of gene activity.
Implications for Genetic Disorders: Fragile X and Rett Syndromes
The implications of Jeannie T. Lee’s research extend far beyond basic science; they address critical aspects of therapies for genetic disorders such as Fragile X Syndrome and Rett Syndrome. Both conditions are linked to mutations on the X chromosome and present significant neurological challenges. By leveraging the insights gained from studying X chromosome inactivation, researchers can explore solutions to restore the expression of healthy genes that are effectively silenced due to the inactivation process. This could dramatically improve the quality of life for those afflicted, indicating a powerful potential for genetic therapies that correct the underlying molecular dysfunction.
Moreover, understanding how inactivated X chromosomes can be effectively ‘unsilenced’ offers hope not just for females but also for males who might be carriers of X-linked mutations. Research supports the notion that by modifying the silencing process, it’s possible to recover gene function in affected cells, which could lead to groundbreaking treatments for a broader range of genetic conditions. As scientists continue to unravel the complexities of XCI, the dream of more targeted therapies to combat genetic disorders seems increasingly attainable.
Therapeutic Strategies from X Inactivation Research
The research into mechanisms of X chromosome inactivation is leading to innovative therapeutic strategies aimed at treating genetic disorders. By understanding how genes are silenced on the X chromosome, scientists can develop methods to selectively reactivate these genes, thus addressing the root causes of conditions like Fragile X and Rett syndromes. The lab’s focus on optimizing compounds that can ‘unsilence’ inactivated genes sets the stage for future clinical trials, potentially transforming how we approach treatment for these disorders. Drugs that can manipulate XCI not only offer hope for affected individuals but also provide a broader framework for addressing various genetic disorders tied to chromosomal anomalies.
Moving forward, the implications of Lee’s findings are profound. By targeting the system that orchestrates X chromosome inactivation, researchers hope to develop more precise interventions that can minimize side effects associated with broader genetic therapies. The challenge lies in developing safe and effective approaches that can re-engage these silenced genes without adversely affecting the normal functioning of adjacent genes. This pursuit not only strengthens the understanding of genetic regulation but paves the way for groundbreaking changes in how we approach the treatment of genetic disorders linked to the X chromosome.
Future Directions in XCI Research
As research on X chromosome inactivation (XCI) progresses, future studies are poised to unveil deeper insights into the molecular mechanisms that govern chromosomal silencing. Scientists like Jeannie T. Lee are increasingly focused on exploring the nuances of how Xist RNA interacts with the Jell-O-like substance surrounding the chromosome, potentially leading to new therapeutic targets. There’s a burgeoning interest in better understanding the genetic and epigenetic factors that contribute to successful XCI, as these could inform how effectively genes can be activated in therapeutic contexts.
The ongoing examination of chromosomal architecture and its association with disease phenotypes will further enhance the potential for developing interventions that can mitigate disorders linked to X-linked mutations. As researchers refine their techniques and expand their knowledge base, we can expect to see advancements in gene therapy that are not only effective but also tailored to the unique challenges posed by conditions like Fragile X Syndrome and Rett Syndrome. The exciting future of XCI research holds the promise of transforming the landscape of genetic disorder treatments.
The Science of Chromosomal Architecture
The architecture of chromosomes plays a crucial role in regulating gene expression and maintaining cellular integrity. Understanding how chromosomes are structured, including their spatial organization and the role of surrounding substances, is essential for unraveling the complexities of diseases involving chromosomal mutations. Scientists are beginning to recognize that just as the structure of a building is critical to its stability, the 3D configuration of chromatin affects how genes are accessed and expressed.
Ongoing research into the science of chromosomal architecture points to a fascinating interplay between genetic components and their environment. By further exploring how external factors influence chromatin structure, researchers can develop strategies that might not just reactivate silenced genes but also correct structural anomalies associated with certain genetic conditions. As these insights deepen, the intersection of genetics and structural biology promises to reveal new avenues for therapeutic intervention in chromosomal disorders.
Innovations in Gene Therapy Approaches
The innovations in gene therapy are rapidly evolving, particularly with the insights gained from research on X chromosome inactivation. By employing techniques that can either reprogram silenced genes or deliver therapeutic agents directly to affected cells, scientists are working toward more effective treatments for genetic disorders such as Fragile X and Rett syndromes. The goal is to harness the body’s own mechanisms for gene activation in a way that is both safe and efficient, minimizing adverse effects while maximizing therapeutic outcomes.
Emerging technologies, such as CRISPR and other genome editing systems, provide a powerful platform for these new therapies to flourish. By precisely targeting the mechanisms that control gene expression, researchers can develop novel solutions that address the underlying causes of genetic disorders. This proactive approach could revolutionize how we treat conditions that have previously been considered difficult or impossible to address, showing that advancements in the understanding of X chromosome biology can lead to groundbreaking changes in clinical practice.
Interdisciplinary Collaboration in Genetic Research
Collaboration across various scientific disciplines is essential for advancing our understanding of complex genetic disorders. In the field of genetics, interdisciplinary efforts that integrate molecular biology, biophysics, computational modeling, and clinical research are yielding promising results. The work of Jeannie T. Lee’s lab exemplifies how collaborative research can unlock new insights into X chromosome inactivation and its implications for diseases like Fragile X Syndrome and Rett Syndrome.
By bringing together experts from diverse backgrounds, scientists can tackle multifaceted questions about gene regulation, chromosomal architecture, and therapeutic development. Such collaboration not only enhances the robustness of findings but also accelerates the process of translating scientific discoveries into clinical applications. As we continue to address the challenges posed by genetic disorders, nurturing interdisciplinary partnerships will be vital to uncover the most effective strategies for treatment and intervention.
Addressing Future Ethical Considerations in Gene Therapy
As the potential for gene therapy emerges from advancements like those achieved through research on X chromosome inactivation, ethical considerations become increasingly critical. The ability to modify gene expression brings with it profound implications for individuals and society at large. Issues such as consent, accessibility, and equitable distribution of therapies must be carefully considered to ensure that the benefits of these advancements are shared broadly, rather than creating disparities based on socioeconomic status.
Moreover, as we develop therapies capable of unsilencing genes linked to genetic disorders, we must remain vigilant about the long-term effects of these interventions. Research must be accompanied by discussions about the responsibility of scientists, clinicians, and policymakers in navigating the implications of genetic modifications. Establishing ethical frameworks will be crucial as we move toward a future where gene therapy becomes more common, ensuring that the technology serves humanity while preserving fundamental ethical principles.
Frequently Asked Questions
What is X Chromosome Inactivation and why is it important in females?
X Chromosome Inactivation (XCI) is a biological process in which one of the two X chromosomes in females is randomly inactivated during early development. This ensures dosage compensation, meaning females have a balanced expression of X-linked genes similar to males, who have only one X chromosome. Understanding XCI is crucial as it plays a significant role in disorders related to the X chromosome, such as Fragile X Syndrome and Rett Syndrome.
How does X Chromosome Inactivation relate to Fragile X Syndrome and Rett Syndrome?
X Chromosome Inactivation is directly related to genetic disorders like Fragile X Syndrome and Rett Syndrome. In these conditions, mutations on the X chromosome can lead to neuronal dysfunction, which in females might be compounded by the inactivation of a functional allele. Research into XCI may lead to novel therapies that can unsilence beneficial genes, potentially alleviating symptoms of these disorders.
What role does the gelatinous ‘Jell-O-like’ substance play in X Chromosome Inactivation?
The ‘Jell-O-like’ substance surrounding chromosomes is essential for X Chromosome Inactivation. This gelatinous material creates a flexible environment that allows the Xist RNA molecule to modify its properties. By changing the surrounding medium, Xist helps the inactivation process by making the X chromosome inaccessible for gene expression, thus contributing to chromosomal silencing.
Can understanding X Chromosome Inactivation lead to treatments for genetic disorders?
Yes, understanding X Chromosome Inactivation has significant therapeutic potential for genetic disorders. By manipulating the inactivation process, researchers aim to unsilence genes on the X chromosome that have been locked away due to inactivation, which is particularly hopeful for conditions like Fragile X Syndrome and Rett Syndrome. This approach could restore gene function and ameliorate symptoms by providing cells access to healthy gene copies.
What potential does unsilencing X-linked genes hold for male patients with Fragile X Syndrome?
Even though males typically do not undergo X Chromosome Inactivation as females do, unsilencing X-linked genes could potentially benefit male patients with Fragile X Syndrome. This is crucial because males can also carry mutations on the X chromosome that could be treated by restoring the function of silenced genes, leading to possible new therapies that address the underlying genetic issues.
How does the process of X Chromosome Inactivation affect gene expression in females?
X Chromosome Inactivation results in the silencing of one X chromosome in females, which serves to equalize gene expression between males and females. This ensures that females, who have two X chromosomes, do not express double the amount of X-linked genes compared to males. The result is a stable balance needed for normal development and function, which is especially relevant in the context of X-linked genetic disorders.
What advancements have been made in X Chromosome Inactivation research recently?
Recent advancements in X Chromosome Inactivation research, particularly from Jeannie T. Lee’s lab, have revealed intricate mechanisms of how Xist RNA interacts with the chromosomal environment. These findings open pathways for therapeutic interventions aiming to unsilence beneficial genes linked to disorders like Fragile X Syndrome and Rett Syndrome, with future clinical trials expected to follow.
How might X Chromosome Inactivation research impact future genetic therapies?
Research into X Chromosome Inactivation has the potential to revolutionize genetic therapies by allowing scientists to target and unsilence specific genes on the X chromosome that are vital for treating conditions like Fragile X Syndrome and Rett Syndrome. By enhancing our understanding of this silencing process, new treatment approaches could emerge that minimize side effects while effectively restoring gene function.
| Key Point | Description |
|---|---|
| X Chromosome Inactivation | Females have two X chromosomes, leading to the need to inactivate one to prevent overexpression of X-linked genes. |
| Role of the Gelatinous Substance | The substance around chromosomes, likened to Jell-O, prevents tangling and facilitates the coating and inactivation of the X chromosome. |
| Xist Function | Xist RNA molecule is responsible for the silencing of the X chromosome by altering the surrounding gelatinous substance. |
| Therapeutic Potential | Research could lead to treatments for genetic disorders like Fragile X and Rett syndrome by unsilencing affected genes. |
| Optimizing Treatments | The lab aims to further optimize approaches and conduct safety studies before moving towards clinical trials. |
Summary
X Chromosome Inactivation is a vital biological process that ensures females with two X chromosomes do not overexpress genes. Recent discoveries by Jeannie T. Lee’s lab reveal the mechanisms behind this inactivation, which involve a gelatinous substance that helps silence genes on one X chromosome. This process not only provides insights into cell biology but also opens doors to potential therapies for genetic conditions associated with the X chromosome, such as Fragile X Syndrome and Rett Syndrome.